BATON ROUGE, La. — The information below is provided by the Louisiana Department of Health. If you have more questions about the coronavirus vaccine, contact your primary healthcare provider.
Why are the COVID-19 vaccines important?
COVID-19 is now the leading cause of death in the United States. More than 7,600 Louisianans died from COVID in 2020 — that’s more than the number of deaths caused by accidents, stroke and diabetes combined in Louisiana in 2017. The vaccines against this virus are a critical tool in ultimately ending the pandemic and getting our lives back to normal.
How is it determined who gets the vaccine?
Everyone in Louisiana will have the opportunity to get vaccinated. The state is looking to the prioritization guidance from the CDC’s Advisory Committee on Immunization Practices (ACIP) and then tailoring that guidance to Louisiana’s context and needs.
Who can get the COVID-19 vaccine now?


The first round of vaccines in Louisiana (Phase 1A) is being given to hospital personnel, residents and staff of nursing home/long-term care facilities, and frontline responders to serve as vaccinators (emergency medical services, fire personnel and law enforcement).
As of January 4, 2021, the vaccine is available in a very limited amount to any and all of the following groups (Phase 1B, Tier 1):
- Persons ages 70 years or older in the community,
- Outpatient clinic providers and clinic staff,
- Urgent care clinic providers and staff,
- Community care clinic providers and staff,
- Behavioral health providers and staff,
- Dialysis providers and recipients,
- Home health service providers and recipients,
- Dental providers and staff, and
- Students, residents, faculty and staff of allied health schools (if not already receiving vaccine or in a plan to receive from their respective schools).
Click HERE for Vaccine Locations.
Patients must contact a participating location and make an appointment at the location. Do not arrive at a location without an appointment. LDH cannot make appointments for residents; only participating locations can.
Who is next in line for the COVID-19 vaccine?


In no particular order, the next groups who will be eligible to receive the vaccine (Phase 1B, Tier 2) are:
- Health-related support personnel (labs, mortuary, pharmacy)
- Essential governmental response personnel
- Judiciary personnel
- Department of Homeland Security personnel, National Guard (non-COVID deployed, federal intelligence and security personnel, military personnel
- First responders not covered in Phase 1A
- Corrections officers and jailers
- Medical transportation services
- Homeless shelter and other congregate group homes/center staff
- Higher education, K-12 school, and daycare personnel
- Food processing and agricultural workers
- Postal personnel
- Public transit workers
- Grocery store workers and other deemed frontline essential workers
LDH will make an announcement when the vaccine becomes available to these groups.
When can the general public get the COVID-19 vaccine?
LDH is following prioritization guidance from ACIP, which recently came out with refined guidance about Phases 1B and 2. LDH continues to review and consider how best to apply this guidance to Louisiana. This is a fluid process, and allocation may evolve depending on the amount of vaccine that is ultimately available to Louisiana. LDH is committed to the equitable distribution and administration of vaccines.
The vaccine is likely to become more widely available for the general population in late spring/summer 2021. When this happens, having a large portion of the population vaccinated is our best shot at a return to some form of normalcy. Based on conversations with our federal partners, we are encouraged that Louisiana will receive enough doses to vaccinate everyone who wants a shot.
How will the COVID-19 vaccine be distributed?
LDH has been working closely with the private and public sector, including pharmacies, hospitals, and nursing homes, in making the vaccine available at locations in both urban and rural communities throughout the state. HHS has also partnered with national pharmacy chains and expects to partner with independent pharmacies and regional chains to ensure access.
Louisiana has also been preparing for COVID vaccination clinics throughout flu season. We have held drive-thru flu shot clinics across the state as a “test run” for the COVID-19 vaccine.
Information about vaccine distribution and administration can change quickly. LDH is committed to transparency about the vaccine, including safety concerns, and will continuously educate the public and address questions the public may have.
Do I need to get more than one dose of the COVID-19 vaccine?
Both the Pfizer and Moderna vaccines are in two doses, administered 3 or 4 weeks apart. You will get the necessary information about the second dose when you get your initial vaccine. The second dose is very important. One dose will not provide long-term protection. People vaccinated with the Pfizer vaccine will receive the second dose 21 days after the first dose. Those getting the Moderna vaccine will receive the second dose 28 days after the first dose.
How much will it cost to get the COVID-19 vaccine?
As a part of the U.S. government’s Operation Warp Speed, the vaccine doses purchased with U.S. taxpayer dollars will be no cost to the person receiving the vaccine. However, providers may charge an administrative fee or for an office visit.

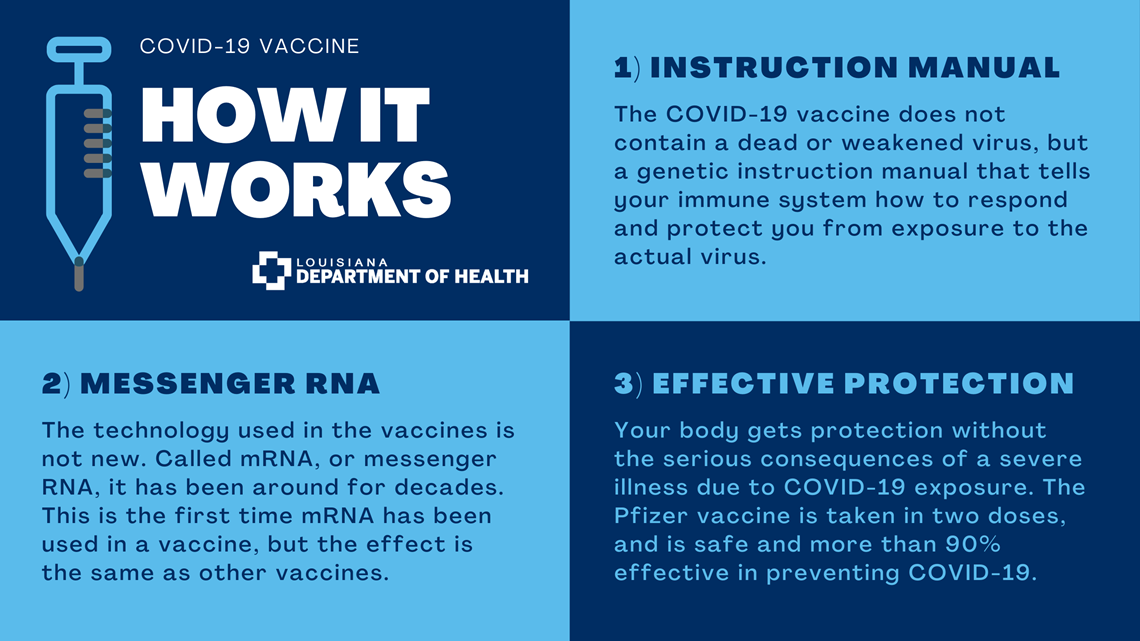
How does the COVID-19 vaccine work?
Unlike many vaccines, the COVID-19 vaccine does not contain a dead or a weakened virus that triggers an immune response. Instead, the COVID-19 vaccine contains a genetic instruction manual that tells your immune system how to respond and protect you from exposure to the actual virus.
The technology used in the vaccines is not new. It is called mRNA, or messenger RNA, and it has been around for decades. This is the first time mRNA has been used in a vaccine, but the effect is the same as other vaccines: Your body gets protection without the serious consequences of a severe illness due to COVID-19 exposure.


Are the COVID-19 vaccines safe?
Vaccines are approved for use by the FDA. The FDA authorization means that trials have proven the vaccine as a safe and effective defense against COVID-19. The FDA and ACIP will continue to monitor safety and effectiveness data.
No steps were skipped during the clinical trials and data review process for COVID-19 vaccines. Safety is a top priority. The COVID vaccines are being held to the same standards as other vaccines to make sure they are safe.
► Get breaking news from your neighborhood delivered directly to you by downloading the new FREE WWL-TV News app now in the IOS App Store or Google Play.

